
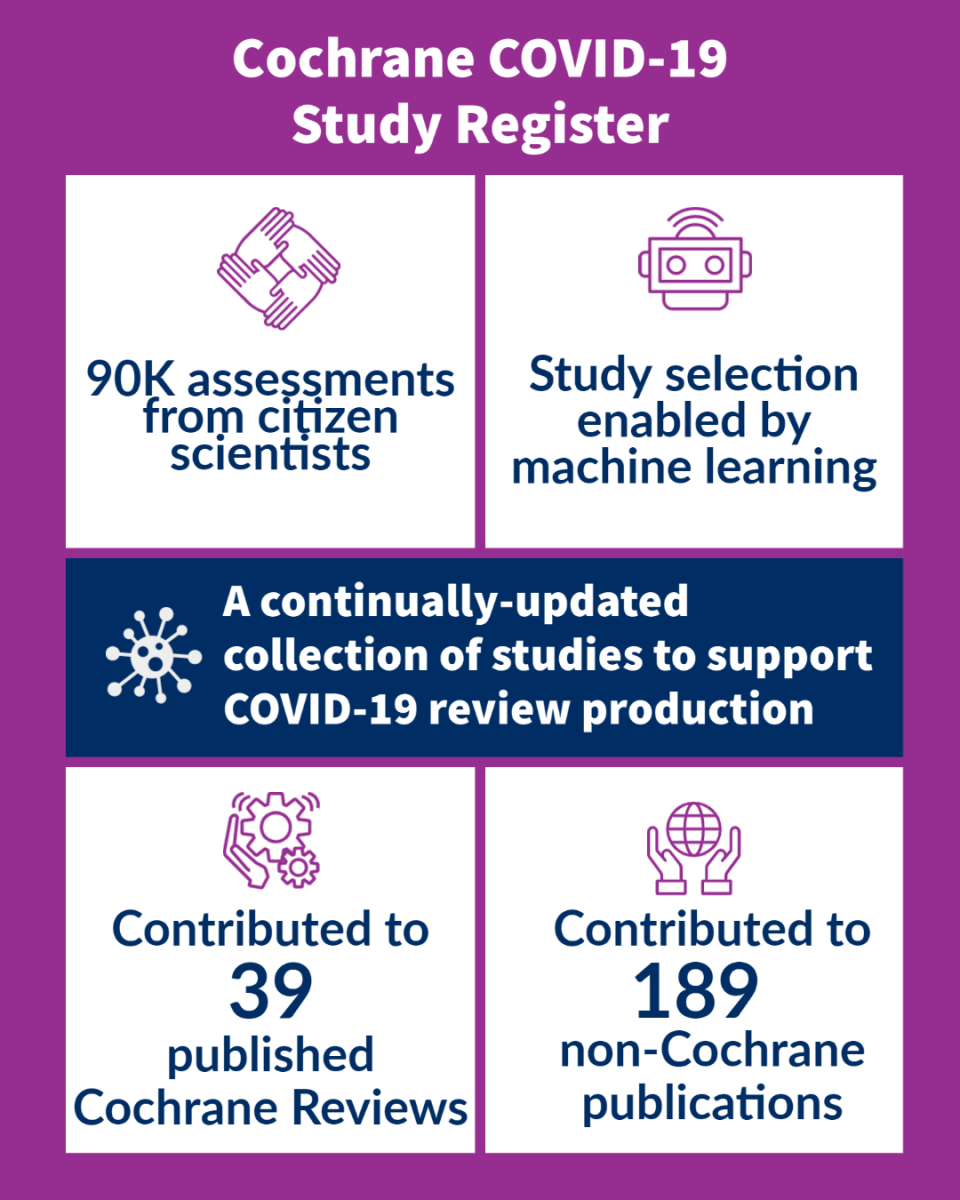
The Cochrane COVID-19 Study Register is a freely-available, continually-updated, annotated reference collection of human primary studies on COVID-19. It launched on April 1, 2020 with 1146 records. After 18 months, the register has grown to nearly 80 times its original size and now includes over 90,000 validated COVID-19 study records! The register has contributed to 39 published Cochrane reviews and 189 non-Cochrane publications.
Making an impact
The CEOsys evidence ecosystem for COVID-19 research is an association of 20 German university hospitals and partner organizations, whose goal it is to compile, summarize, and assess the certainty of results coming from scientific studies examining the most pressing questions about prevention, treatment, and consequences of COVID-19. Based on this evidence, the project creates recommendations for action. Systematic review producer and Cochrane review co-author, Ina Monsef, reflects on the Cochrane COVID-19 Study Register’s contribution to the CEOsys:
The Cochrane COVID-19 Study Register is a primary source of evidence for reviews produced within the CEOsys project. Using this curated register saved the whole team time. Information specialists, methodologists, and clinicians - we all benefited from less information noise. The register contains all important primary sources. The ability to filter by study design allowed us to quickly select the relevant studies for each review question. Another helpful feature is the study type filter for platform trials which allowed us to find updated information about these important studies. Over the course of the COVID-19 pandemic, the Cochrane register has been a valuable tool to help us produce high-quality COVID-19 reviews rapidly.
-Ina Monsef, Information Specialist Cochrane Haematology, University Hospital of Cologne, CEOsys Study Identification Team
Citizen Science and Machine learning
To maintain its massive production volume, the Cochrane COVID-19 Study Register relies on innovations in machine learning and Cochrane’s citizen science platform, Cochrane Crowd. With colleagues from the EPPI-Centre, we implemented a study classifier in January to help reduce our screening workload by 25%. The process to develop the study classifier will be published shortly and is currently available as a Research Square preprint.
Similar to the publishing processes used for the Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane uses a combination of automated centralised searches, machine learning classifiers and Crowd assessments to build the Cochrane COVID-19 Study Register. Since launching the Cochrane Crowd task COVID Quest! in June 2020, volunteer contributors have made over 90,000 assessments to the register.
Make your contribution to the Cochrane COVID-19 Study Register
To help us celebrate 100,000 assessments, please join us on World Evidence-Based Healthcare Day on October 20th for a 12 hour screening challenge in COVID Quest! (08:00-20:00 BST). Participate at any point during the challenge for a chance to win prizes. You can also contribute to COVID Quest! at any time - there are always studies waiting for you to screen and classify. Anyone can join Cochrane Crowd and every contribution helps. No previous experience is necessary!


